I-Enterovirus Universal, i-EV71 kanye ne-CoxA16
Igama Lomkhiqizo
Ikhithi Yokuthola I-HWTS-EV026B-Enterovirus Universal, EV71 kanye ne-CoxA16 Nucleic Acid (i-Fluorescence PCR)
I-HWTS-EV020Y/Z-Ikhithi Yokuthola I-Enterovirus Universal, EV71 kanye ne-CoxA16 Nucleic Acid (i-Fluorescence PCR) eyomisiwe ngokuqandisa
Isitifiketi
I-CE/MDA(HWTS-EV026)
I-Epidemiology
Isifo somlomo wesandla (i-HFMD) yisifo esivame kakhulu esithathelwanayo ezinganeni. Sivame ukwenzeka ezinganeni ezingaphansi kweminyaka emi-5 ubudala, futhi singabangela i-herpes ezandleni, ezinyaweni, emlonyeni nakwezinye izingxenye, kanti inani elincane lezingane lingabangela izinkinga ezifana ne-myocarditis, i-pulmonary edema, i-aseptic meningoencephalitis, njll. Izingane ngazinye ezinezifo ezinkulu ziyawohloka ngokushesha futhi zithambekele ekufeni uma zingelashwa ngokushesha.
Njengamanje, kutholakale izinhlobo ezingu-108 zama-enterovirus, ahlukaniswe ngamaqembu amane: A, B, C kanye no-D. Ama-enterovirus abangela i-HFMD ahlukene, kodwa i-enterovirus 71 (EV71) kanye ne-coxsackievirus A16 (CoxA16) yizona ezivame kakhulu futhi ngaphezu kwe-HFMD, angabangela izinkinga ezinkulu zesistimu yezinzwa ephakathi njenge-meningitis, i-encephalitis, kanye nokukhubazeka okukhulu kwe-flaccid.
Isiteshi
| I-FAM | I-Enterovirus |
| I-VIC (HEX) | I-CoxA16 |
| I-ROX | I-EV71 |
| CY5 | Ukulawula kwangaphakathi |
Amapharamitha Obuchwepheshe
| Isitoreji | Uketshezi: ≤-18℃ EbumnyameniUkwenziwa kwe-Lyophilization: ≤30℃ |
| Isikhathi sokuphelelwa yisikhathi | Uketshezi: izinyanga ezingu-9Ukuvuselelwa kwe-Lyophilization: izinyanga ezingu-12 |
| Uhlobo Lwesifanekiso | Isampula ye-throat swab, i-Herpes fluid |
| Ct | ≤38 |
| CV | ≤5.0% |
| I-LoD | Amakhophi angu-500/mL |
| Izinsimbi Ezisebenzayo | Ingafanelana namathuluzi e-PCR e-fluorescent ajwayelekile emakethe.Izinhlelo ze-PCR ze-ABI 7500 Zesikhathi Sangempela Izinhlelo ze-PCR ze-ABI 7500 ze-Fast Real-Time Izinhlelo ze-PCR Zesikhathi Sangempela ze-SLAN-96P Izinhlelo ze-PCR ze-QuantStudio®5 Zesikhathi Sangempela Izinhlelo ze-PCR ze-LightCycler®480 Zesikhathi Sangempela Izinhlelo Zokuthola i-LineGene 9600 Plus Real-Time PCR I-MA-6000 Real-Time Quantitative Thermal Cycler Uhlelo lwe-BioRad CFX96 Real-Time PCR Uhlelo lwe-BioRad CFX Opus 96 lwe-Real-Time PCR |
Isixazululo se-PCR Esiphelele
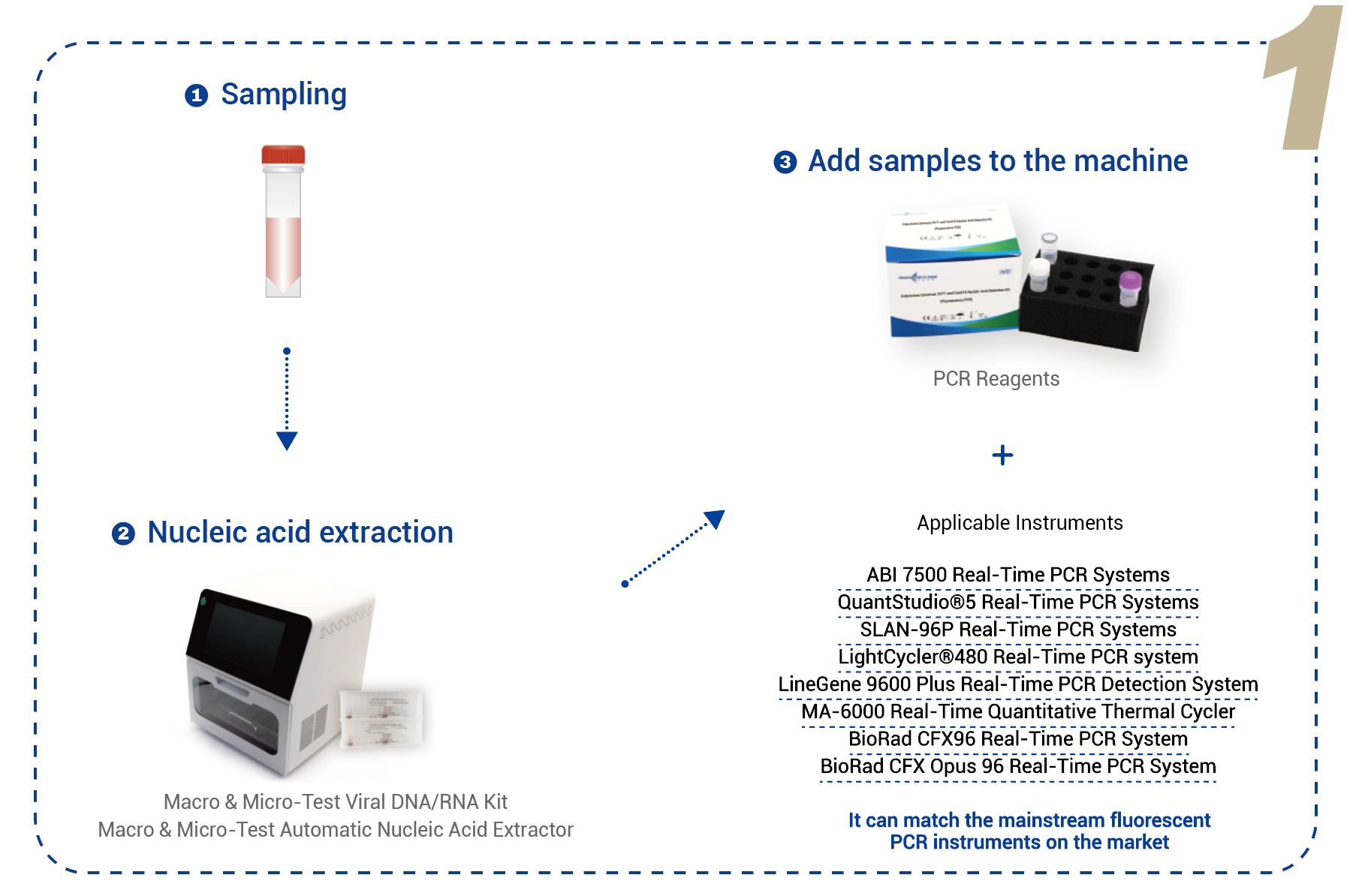

I-reagent yokukhipha enconyiwe: I-Macro & Micro-Test Viral DNA/RNA Kit (HWTS-3017) (engasetshenziswa ne-Macro & Micro-Test Automatic Nucleic Acid Extractor (HWTS-EQ011)) yi-Jiangsu Macro & Micro-Test Med-Tech Co., Ltd. Ukukhipha kufanele kwenziwe ngokwencwadi yemiyalelo. Umthamo wesampula okhishwe ungama-200μL, kanti umthamo wokususa onconyiwe ungama-80μL.
I-reagent yokukhipha enconywayo: I-Macro & Micro-Test Sample Release Release Reagent (HWTS-3005-8). Ukukhipha kufanele kwenziwe ngokwencwadi yemiyalelo. Amasampula okukhipha angama-oropharyngeal swabs noma amasampula e-herpes fluid eziguli eziqoqwe endaweni. Faka ama-swabs aqoqwe ngqo ku-Macro & Micro-Test Sample Release Release Reagent, i-vortex bese uxuba kahle, ubeke ekamelweni lokushisa imizuzu emi-5, uyikhiphe bese uyiguqula bese uxuba kahle ukuze uthole i-RNA yesampula ngayinye.
I-reagent yokukhipha enconyiwe: I-QIAamp Viral RNA Mini Kit (52904) yi-QIAGEN noma i-Nucleic Acid Extraction noma i-Purification Reagent (YDP315-R). Ukukhipha kufanele kwenziwe ngokuhambisana ngokuqinile nencwadi yemiyalelo.