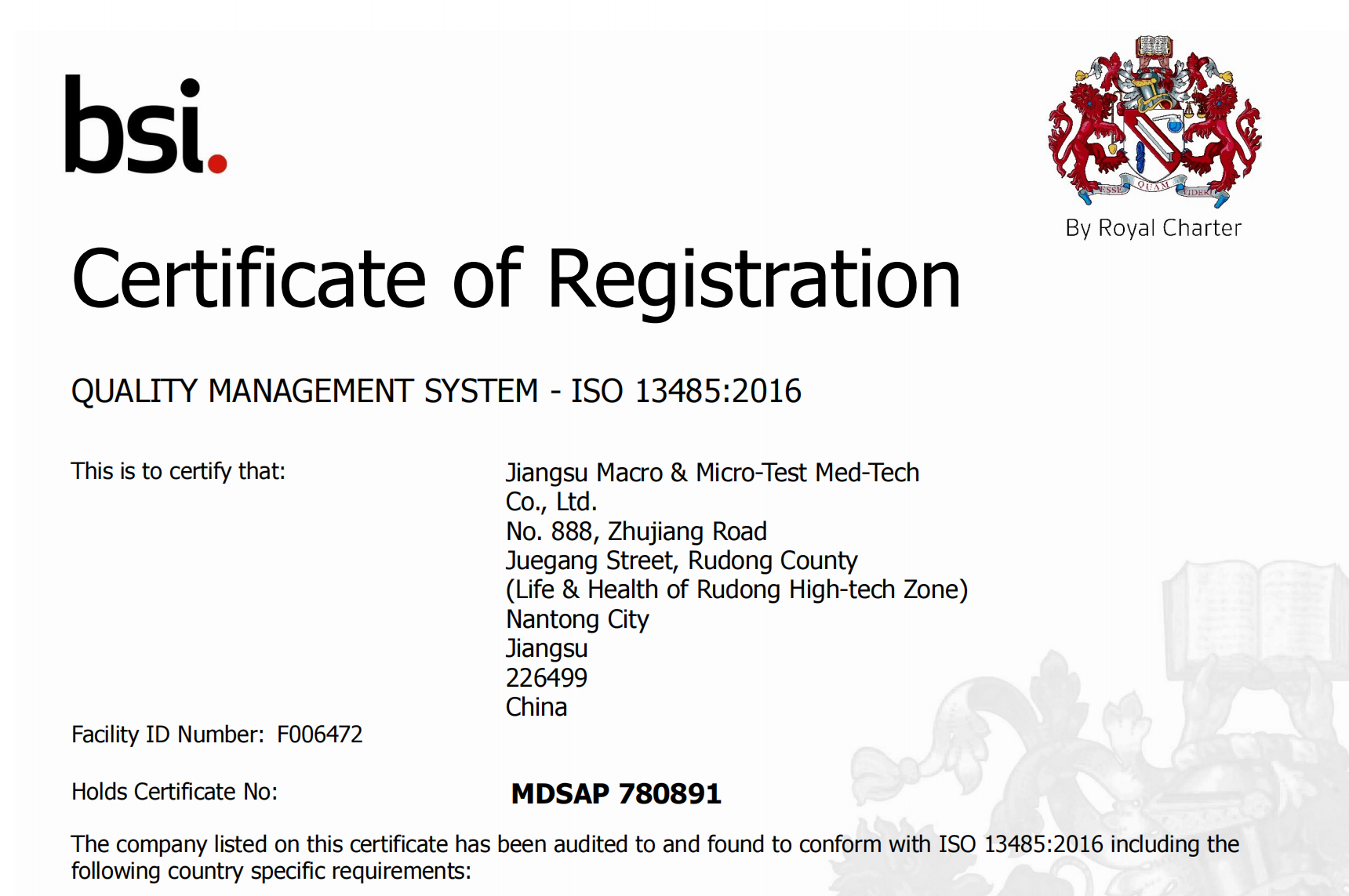
Siyajabula ukumemezela ukuthola isitifiketi se-Medical Device Single Audit Program (#MDSAP). I-MDSAP izosekela ukuvunyelwa kwezentengiselwano kwemikhiqizo yethu emazweni amahlanu, okuhlanganisa i-Australia, iBrazil, iCanada, iJapan kanye ne-US.
I-MDSAP ivumela ukuqhutshwa kokuhlolwa okukodwa kokulawula kohlelo lokuphathwa kwekhwalithi lomkhiqizi wedivayisi yezokwelapha ukuze kwaneliswe izidingo zezindawo eziningi zokulawula noma iziphathimandla ezivumela ukulawulwa okufanele kwezinhlelo zokuphathwa kwekhwalithi zabakhiqizi bedivayisi yezokwelapha ngenkathi kunciphisa umthwalo wokulawula embonini. Uhlelo okwamanje lumelela i-Australia's Therapeutic Goods Administration, i-Brazil's Agência Nacional de Vigilância Sanitária, i-Health Canada, i-Japan's Ministry of Health, Labour and Welfare kanye ne-Pharmaceutical and Medical Devices Agency, kanye ne-US Food and Drug Administration's Center for Devices and Radiological Health.
Isikhathi sokuthunyelwe: Ephreli-13-2023